Step 5 of 18
Atrioventricular canal (AVC) defects
An AV canal defect is an absence of septation in the centre of the heart, which is very frequently associated with Down syndrome (60-86% of cases) [3]. It accounts for 4% of all congenital heart diseases, but also occurs in other pathologies such as double-outlet right ventricle (DORV) or subaortic stenosis. In countries with the appropriate technology, it is often diagnosed prior to birth. The AVC defect consists of an ostium primum-type ASD, an inlet VSD, and a single 5-leaflet atrioventricular valve surrounding a central orifice. The tricuspid and mitral valves are typically located in the same plane – they have variable insertions between each other and with the crest of the interventricular septum (Videos and Figure 14.40).
Video: 4-chamber view of an AV canal, with typical insertion of tricuspid and mitral valves on the same plane at the level of the septum; presence of an ASD ostium primum.
Video: 4-chamber view of an AV canal, with typical insertion of tricuspid and mitral valves on the same plane at the level of the septum; presence of a VSD at the level of the admission chamber.
In addition to ASDs and VSDs, the AV canal defect encompasses a variety of malformations of the tricuspid and mitral valve with various degrees of regurgitation through a central cleft (Video).
Video: 4-chamber view of an AV canal with colour flow, showing a double mitral regurgitation (through the commissure and through the cleft near the septum), and a tricuspid regurgitation.
These orifices communicate between each other, and shunting may occur in numerous directions – LV→RV, LV→RA, LA→RA, LA→RV – combined with mitral insufficiency (LV→LA) and tricuspid insufficiency (RV→RA) through the valve clefts. An inherent feature of this central septation defect and the apical displacement of the mitral annulus is the highly anterior positioning of the aorta, which narrows the LV outflow tract (LVOT), causes it to adopt a gooseneck deformity, and renders it highly susceptible to dynamic obstruction. The posterior shift of the atrioventricular annulus results in a narrowing of the LV inlet and a shortening of its outflow, bringing the papillary muscles laterally closer together [5]. In some cases, the AVC defect is partial, with just one ostium primum-type ASD or one inlet VSD [4].

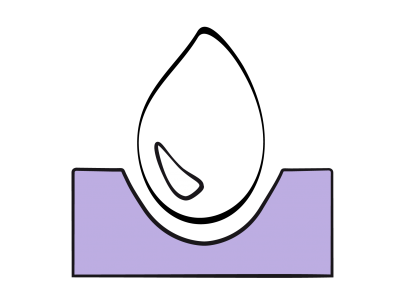
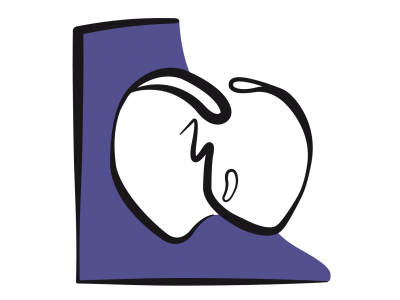
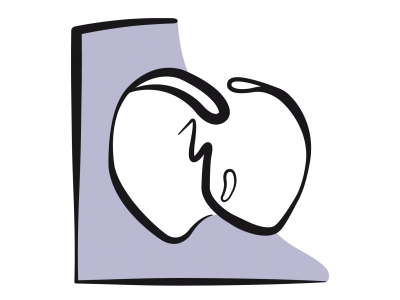
Figure 14.40: Complete AV canal defect with ASD, VSD, and median cleft in the septal leaflets of the tricuspid and mitral valves. The arrows represent the various shunts and simultaneous regurgitations.
Clinically, the AVC defect presents as a large L-to-R shunt with an increase in pulmonary blood flow and volume overload, both for the RV via the ASD and for the LV via the VSD. Symptoms occur in the first months of life and are determined by the size of the VSD. Pulmonary arterial hypertension (PAH) develops early in disease progression if a major VSD is present. This secondarily creates pressure overload for the RV. In cases of partial AVC with no VSD, the ASD prompts a volume overload for the RV and the right-sided circulation. Such cases are managed in the same way as simple ASDs.
In the event of a complete AV canal defect, surgical correction is performed between the age of 3 and 6 months in order to avoid PAH complications secondary to the VSD. It involves closing the ASD and VSD with one or two patches, repairing cleft leaflets, and avoiding conduction paths, which are often abnormally positioned in subjects with this anomaly. The surgical approach is through the RA. In the event of a partial AV canal defect (ostium primum ASD), correction may be delayed until the age of 2 to 5 years, unless congestive ventricular failure develops [1]. An echocardiographic examination is necessary to check the leak-tightness of patches, the valve reconstructions and the anatomy of the LVOT. A comparison of saturation levels in the IVC and SVC with those of the PA gives an indication of the extent of any residual shunting. Surgical mortality varies from 1% for partial AV canal defects to 5% for complete AV canal defects [6]. Postoperative complications are: third degree AV block, PAH crisis, right or left ventricular failure, and residual valvular leakage of the tricuspid or mitral valve. In the rare cases that are inoperable with CPB, palliation is performed by banding the pulmonary artery to limit PAH.
Anaesthesia
The technique is selected according to the extent of L-to-R shunting and to the patient's clinical condition. In the event of ventricular failure and PAH, intravenous fentanyl/midazolam-based induction is required. Otherwise, an induction with a halogenated agent (sevoflurane) is possible.
Video: 4-chamber view of an AV canal, with typical insertion of tricuspid and mitral valves on the same plane at the level of the septum; presence of an ASD ostium primum.
Video: 4-chamber view of an AV canal, with typical insertion of tricuspid and mitral valves on the same plane at the level of the septum; presence of a VSD at the level of the admission chamber.
In addition to ASDs and VSDs, the AV canal defect encompasses a variety of malformations of the tricuspid and mitral valve with various degrees of regurgitation through a central cleft (Video).
Video: 4-chamber view of an AV canal with colour flow, showing a double mitral regurgitation (through the commissure and through the cleft near the septum), and a tricuspid regurgitation.
These orifices communicate between each other, and shunting may occur in numerous directions – LV→RV, LV→RA, LA→RA, LA→RV – combined with mitral insufficiency (LV→LA) and tricuspid insufficiency (RV→RA) through the valve clefts. An inherent feature of this central septation defect and the apical displacement of the mitral annulus is the highly anterior positioning of the aorta, which narrows the LV outflow tract (LVOT), causes it to adopt a gooseneck deformity, and renders it highly susceptible to dynamic obstruction. The posterior shift of the atrioventricular annulus results in a narrowing of the LV inlet and a shortening of its outflow, bringing the papillary muscles laterally closer together [5]. In some cases, the AVC defect is partial, with just one ostium primum-type ASD or one inlet VSD [4].
Figure 14.40: Complete AV canal defect with ASD, VSD, and median cleft in the septal leaflets of the tricuspid and mitral valves. The arrows represent the various shunts and simultaneous regurgitations.
Clinically, the AVC defect presents as a large L-to-R shunt with an increase in pulmonary blood flow and volume overload, both for the RV via the ASD and for the LV via the VSD. Symptoms occur in the first months of life and are determined by the size of the VSD. Pulmonary arterial hypertension (PAH) develops early in disease progression if a major VSD is present. This secondarily creates pressure overload for the RV. In cases of partial AVC with no VSD, the ASD prompts a volume overload for the RV and the right-sided circulation. Such cases are managed in the same way as simple ASDs.
In the event of a complete AV canal defect, surgical correction is performed between the age of 3 and 6 months in order to avoid PAH complications secondary to the VSD. It involves closing the ASD and VSD with one or two patches, repairing cleft leaflets, and avoiding conduction paths, which are often abnormally positioned in subjects with this anomaly. The surgical approach is through the RA. In the event of a partial AV canal defect (ostium primum ASD), correction may be delayed until the age of 2 to 5 years, unless congestive ventricular failure develops [1]. An echocardiographic examination is necessary to check the leak-tightness of patches, the valve reconstructions and the anatomy of the LVOT. A comparison of saturation levels in the IVC and SVC with those of the PA gives an indication of the extent of any residual shunting. Surgical mortality varies from 1% for partial AV canal defects to 5% for complete AV canal defects [6]. Postoperative complications are: third degree AV block, PAH crisis, right or left ventricular failure, and residual valvular leakage of the tricuspid or mitral valve. In the rare cases that are inoperable with CPB, palliation is performed by banding the pulmonary artery to limit PAH.
Anaesthesia
The technique is selected according to the extent of L-to-R shunting and to the patient's clinical condition. In the event of ventricular failure and PAH, intravenous fentanyl/midazolam-based induction is required. Otherwise, an induction with a halogenated agent (sevoflurane) is possible.
- Minimise myocardial depression.
- Lower SVR, which reduces shunting and ventriculo-atrial regurgitation.
- Reduce excessive pulmonary blood flow by increasing PVR (normo- or hypercapnia, PEEP).
- In the event of PAH, avoid any factors that stimulate PVR (stress, pain, hypothermia, etc.) and actively reduce PVR (hyperventilation, alkalosis, high doses of fentanyl, NO•).
- Intubation: may be difficult if the AVC defect is combined with other conditions such as Down syndrome.
- Post-CPB, inotropic and vasodilator support is required (dobutamine, milrinone-epinephrin). Pulmonary vascular reactivity is often exacerbated. Children are kept deeply anaesthetised and hyperventilated. NO• is used liberally.
- Postoperative monitoring: pulmonary catheter (continuous SvO2), catheter in the LA.
Down's syndrome, which is associated with over half of all AVC defect cases, has considerable implications for anaesthesia: intubation difficulties, hypothyroidism, frequent PAH, tendency for bradycardia, heightened perioperative inflammatory response, risk of laryngeal oedema on extubation. Complications are also more common: pulmonary superinfection, post-extubation dyspnoea, PAH and right-sided failure, complete AV block, residual mitral and/or tricuspid insufficiency, residual shunting, dynamic stenosis of the LVOT.
Long-term complications of surgical correction performed on young children are: persistent mitral regurgitation, residual leakage through the patch, dynamic subaortic stenosis, and complete AV block requiring a permanent pacemaker. The rate of reoperation following an AVC correction performed in infancy is very high (11-32%) [2]. The most common causes of reoperation are mitral or tricuspid insufficiency, residual L-to-R shunting, obstruction of the LVOT (mean gradient > 50 mmHg) and LV dysfunction. The long-term survival rate post-reoperation is 88% [2].
Long-term complications of surgical correction performed on young children are: persistent mitral regurgitation, residual leakage through the patch, dynamic subaortic stenosis, and complete AV block requiring a permanent pacemaker. The rate of reoperation following an AVC correction performed in infancy is very high (11-32%) [2]. The most common causes of reoperation are mitral or tricuspid insufficiency, residual L-to-R shunting, obstruction of the LVOT (mean gradient > 50 mmHg) and LV dysfunction. The long-term survival rate post-reoperation is 88% [2].
| Atrioventricular canal (AVC) defects |
|
Lack of central coalescence of the cardiac septa leading to:
- Ostium primum ASD - Inlet VSD - Cleft in the septal leaflet of the tricuspid valve and in the anterior leaflet of the mitral valve - LVOT deformity - Complex shunting between LA-RA, LV-RV, LA-RV - Tricuspid and mitral insufficiencies The VSD causes volume overload for the LV. In partial AV canal defects (VSD absent), the ASD causes a volume overload for the RV. If a major VSD is present: high risk of PAH and secondary RV dysfunction. If the VSD is minimal, progression is the same as for an ASD. If wide cleft in the anterior leaflet, progression is the same as for mitral insufficiency. Management: increase PVR and lower SVR – if PAH, lower PVR by hyperventilation, NO, alkalosis, fentanyl. Inotropic support often necessary Anaesthesia technique selected based on the primary constraint: L-to-R shunting, PAH, ventricular failure, or mitral/tricuspid regurgitation. Postoperative complications: AV block, PAH crisis, right or left ventricular failure, residual valve or patch leakage, subobstruction of the LVOT. |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- BENT ST. Anesthesia for left-to-right shunt lesions. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 297-327
- HOOHENKERK GJ, BRUGGEMANS EF, KOOLBERGEN DR, et al. Long-term results of reoperation for left atrio-ventricular valve regurgitation after correction of atrio-ventricular septal defects. Ann Thor Surg 2012; 93:849-55
- LANGE R, GUENTHER T, BUSCH R, et al. The presence of Down syndrome is not a risk factor in complete atrioventricular septal defect repair. J Thorac Cardiovasc Surg 2007; 134:304-10
- PICCOLI GP. Morphology and classification of complete atrioventricular defects. Brit Heart J 1979; 42:633-9
- SNIDER AR, SERWER GA, RITTER SB. Defects in cardiac septation. In : SNIDER AR, SERWER GA, RITTER SB. Echocardiography in pediatric heart disease. 2nd ed. St Louis, Mosby, 1997, pp. 235-96.
- STARK J. GALLIVAN S, LOVEGROVE J, et al. Mortality rates after surgery for congenital heart defects in children and surgeon’s performance. Lancet 2000; 355:1004-7
14. Anesthesia for paediatric heart surgery
- 14.1 Introduction
- 14.2 Pathophysiology
- 14.3 Haemodynamic strategies
- 14.3.1 Classification
- 14.3.2 Left-to-right shunt and high pulmonary blood flow
- 14.3.3 Pulmonary hypertension in children
- 14.3.4 Cyanotic right → left shunt and reduced pulmonary blood flow
- 14.3.5 Cyanotic right → left shunt and reduced systemic blood flow
- 14.3.6 Bidirectional cyanotic shunt
- 14.3.7 Heart diseases without shunting: obstructions and valvular heart diseases
- 14.3.8 Treatment options for neonates
- 14.3.9 Drug therapy
- 14.4 Anaesthetic technique
- 14.5 CPB in children
- 14.6 Anaesthesia for specific pathologies
- 14.6.1 Introduction
- 14.6.2 Anatomical landmarks
- 14.6.3 Anomalous venous returns
- 14.6.4 Atrial septal defects (ASDs)
- 14.6.5 Atrioventricular canal (AVC) defects
- 14.6.6 Ebstein anomaly
- 14.6.7 Anomalies of the atrioventricular valves
- 14.6.8 Ventricular septal defects (VSDs)
- 14.6.9 Ventricular hypoplasia
- 14.6.10 Tetralogy of Fallot
- 14.6.11 Double outlet right ventricle (DORV)
- 14.6.12 Pulmonary atresia
- 14.6.13 Anomalies of the left ventricular outflow
- 14.6.14 Transposition of the great arteries (TGA)
- 14.6.15 Truncus arteriosus
- 14.6.16 Coarctation of the aorta
- 14.6.17 Arterial abnormalities
- 14.6.18 Heart transplantation
- 14.7 Conclusions