Step 6 of 9
Anaesthetic technique
Choosing a technique is less important than fully understanding the patient’s specific haemodynamics. The first step is to gain adequate knowledge of echocardiography, MRI, and where appropriate, catheterisation data. It is important to check for data on any previous operations or associated malformations. It is essential to determine SpO2 at room air in the preoperative phase, free from the effects of any drugs. Congenital heart diseases must be considered as systemic diseases potentially affecting all systems. As such, some malformations may be combined with deformities of the connective tissue and skeleton that may create issues for intubation. They should ideally be identified in advance.
Preoperative examinations
Routine checks include an ECG, chest X-ray and the customary laboratory tests including blood coagulation tests. Arterial oximetry (SaO2, SpO2) provides the most vital information, since it reflects the drop in pulmonary blood flow and the degree of R-to-L shunting. These data are supplemented by various specific examinations (Table 15.2) [3,4,10].
Preoperative examinations
Routine checks include an ECG, chest X-ray and the customary laboratory tests including blood coagulation tests. Arterial oximetry (SaO2, SpO2) provides the most vital information, since it reflects the drop in pulmonary blood flow and the degree of R-to-L shunting. These data are supplemented by various specific examinations (Table 15.2) [3,4,10].
- Echocardiography: this is an essential tool. It provides information on basic anatomy and on the morphology of the heart chambers and valves and their function. The Doppler measures blood flows, gradients, shunts and, indirectly, RV and/or PA pressure (see Calculation of right-sided pressure). Transesophageal echography may also be performed for greater precision with regard to delicate or posterior structures. Echocardiography is less effective for assessing the RV and inappropriate for examining extra-cardiac vessels.
- MRI: provides a very detailed three-dimensional reconstruction of the anatomy and is superior to an echocardiogram in terms of assessing the RV, biventricular function, chamber volume, tissue characteristics and extra-cardiac vessels (pulmonary veins, aorta, collaterals). It can also be used to measure flows and calculate shunts, gradients or regurgitation fraction.
- CT-scan: more widely available and faster than MRI, offering excellent anatomical definition of structures, particularly those that are extra-cardiac. However, its drawback is the use of ionising radiation and contrast agents. Multislice CT angiography can be used to screen for coronary lesions.
- Angiocatheterisation: this is used solely in unclear cases to define extra-cardiac vessels and accurately measure right-sided pressure (PAP, PVR) and pulmonary vascular reactivity (NO test). In cardiac surgery, coronary arteriography is indicated in men aged > 40 years and menopausal women.
Exercise tolerance is assessed based on the patient's medical history and by ergometry with measurement of maximal O2 uptake. Functional capacity is the most important factor when deciding when to perform surgery.

Premedication
Hypoventilation and hypoxia are catastrophic in patients with low pulmonary blood flow or a cyanotic shunt. Premedication must therefore be moderate and not involve opiates. Preoperative fasting may cause dehydration in cyanotic patients whose Ht is high and whose blood hyperviscosity is an additional risk factor. These patients require a fluid infusion if they undergo surgery in the second part of the day or in very warm conditions. Grown-up congenital heart patients are highly dependent on their family and medical team. They are prone to panic in unknown environments or those perceived as hostile and stressful. This phenomenon affects Down syndrome patients in particular. It is therefore important to show them considerable empathy and ensure that they are unaware of the stress that their pathology causes the medical team.
Equipment
Vascular access points are often limited by previous operations and anatomical constraints. For example, arterial pressure is not measurable in the arm on the side of a Blalock-Taussig shunt, there is no venous access to the right heart after a Fontan procedure, and the IVC is often interrupted in heterotaxy syndrome. Compulsory precautions against the accidental injection of air into venous lines must be taken for any patients with shunts: aspiration of blood prior to injection, 3-way valves on all tubing, deairing of residual bubbles from syringes, injection with the syringe in a vertical position to ensure that bubbles remain attached to the plunger (Vidéo CD 3.0 Fig 15-58).
Monitoring
SpO2 is an extremely valuable indicator for congenital heart disease patients. A good signal must therefore be sought and the transducer must be positioned appropriately for the pathology. Variations in SVR and PVR, actual pulmonary blood flow and Qp/Qs ratio are clearly reflected in variations in arterial oxygen saturation (SaO2, SpO2) or tissue oxygen saturation (ScO2, cerebral oxygen saturation measured by near-infrared spectroscopy). However, these measurements provide no information on O2 delivery in the event of a large L-to-R shunt (Qp/Qs ≥ 3:1). In the event of R-to-L shunting, ETCO2 gives an underestimation of PaCO2 due to the dead-space effect of blood volume avoiding the lungs.
An invasive measurement of arterial pressure is the second most important monitoring procedure [5]. It provides instant data on SVR, blood volume and systolic function. Moreover, it enables frequent blood-gas analyses and rapid detection of metabolic acidosis. It is essential to set it up prior to induction.
Central venous access may be difficult in the event of a left-sided superior vena cava or atrial switch after TGA. Following a Glenn procedure, the catheter placed in the jugular or subclavian vein measures pressure in the right pulmonary artery. CVP is measured by femoral line. If the venous return is diverted to the PA as in the Fontan procedure, a central venous catheter actually measures the upstream pressure of the pulmonary circulation, which must be at least 15 mmHg. In these highly thrombogenic situations, the catheter must be left in place for as short a period as possible. Moreover, it is impossible to interpret filling pressures in terms of blood volume due to congenital heart disease patients’ highly unusual haemodynamic conditions. CVP or PAWP are not useful for determining whether patients are hypo- or hypervolaemic.
The Swan-Ganz pulmonary catheter is not the most important tool and its contribution is somewhat disappointing for congenital heart disease patients, as it only analyses the pulmonary circulation. However, pulmonary blood flow may be different to systemic blood flow (Qp/Qs ≠ 1). Although PAP measurement provides reassurance, it is rarely indicated except in cardiac surgery cases with pulmonary hypertension. Filling pressures are even less effective blood volume end points in congenital heart disease patients than in normal individuals, since they are primarily dependent on anatomopathological conditions. It may be impossible or dangerous to position the catheter in many cases: atrial switch (Mustard or Senning procedure), tight pulmonary stenosis (tetralogy of Fallot), Fontan circulation (no RV). If an ASD or R-to-L shunt is present, the catheter may switch to the left. Moreover, shunting or atrioventricular valve insufficiency skews calculations of cardiac output and of resistance. The situation is unclear in the event of significant tricuspid insufficiency (TI). It is generally accepted that transpulmonary thermodilution gives an underestimation of actual cardiac output in the event of TI. However, this only appears to hold true in situations with high blood flow, since the TI causes overestimation of CO in the event of low blood flow [9]. CO is also overestimated by thermodilution in the event of L-to-R shunting. Since pulmonary hypertension is generally combined with TI, Swan-Ganz gives an underestimation of CO if PAP is high.
The PiCCO system avoids pitfalls associated with a pulmonary catheter by assessing systemic blood flow. However, transpulmonary thermodilution is required in order to calibrate it and its calculation of cardiac output is also skewed by any shunting present. It is particularly useful postoperatively following cardiac surgery, i.e. after shunts have been corrected. If shunting is present, the Qp/Qs ratio, which is assessed by SaO2 and acid-base balance, is more significant than the absolute blood flow value.
Perioperative transesophageal echocardiography (TEE) is without doubt a less invasive and much more effective monitoring technique. It provides abundant data on patients’ anatomy, remodelling, pathophysiology, ventricular function and blood volume, which is particularly useful since filling pressure measurements are unreliable for assessing blood volume in congenital heart disease patients (see below Impact of TEE).
Cerebral monitoring techniques such as EEG, BIS, transcranial Doppler and cerebral O2 saturation (ScO2, NIRS Near-infrared spectroscopy) are important if deep hypothermia or total circulatory arrest are required for surgery. Detailed information regarding these procedures can be found in Chapter 18 (see Anaesthesia for aortic arch surgery, Monitoring) and is not provided here.
Induction
Ideally, a Valsalva manoeuvre should be performed prior to induction in order to test the impact of positive-pressure ventilation on arterial pressure (catheter in place before induction). Direct flow from the systemic venous return to the arterial line accelerates induction with intravenous agents if a R-to-L shunt is present. In contrast, induction and elimination of halogenated agents are slowed due to low pulmonary blood flow [11]. In the event of L-to-R shunting, induction with intravenous agents is slightly slowed. The effect on induction with halogenated agents is negligible [12]. In practice, such notions have little impact on the course of anaesthesia.
Induction agents are chosen based on the pathology's haemodynamic impact. However, many are associated with effects that render their use with congenital heart disease patients problematic: reduction of preload and SVR (propofol, midazolam), negative inotropic effect (thiopental, propofol), tachycardia (thiopental) [1]. Etomidate is without doubt the substance with the least harmful effects and is recommended for any complex cardiac anaesthesia. However, a fentanyl-midazolam combination is a good alternative. Nevertheless, midazolam considerably delays extubation unless it is used solely for sedation (1-3 mg). Fentanyl and sufentanil are the most stable and best controlled opiates. They dose-dependently block stress response and inhibit PAH crises. Although remifentanil is useful for brief procedures, the bradycardia it causes may be prohibitive. While ketamine is useful for children, it is inappropriate for adult haemodynamic conditions since it increases PVR and myocardial O2 requirement, and has a significant direct cardiodepressant effect, which is concealed by its sympathetic stimulation [13].
Anaesthetic procedure (Table 15.8)
It is simplest to maintain anaesthesia with a halogenated agent. Sevoflurane offers the greatest stability. Isoflurane is indicated for lowering SVR. Desflurane is contraindicated due to its sympathetic stimulation and its idiosyncrasy of causing an increase in PVR if the inspired concentration is changed [6,7]. Ketamine and N2O have a pulmonary hypertensive effect in adults. Deep anaesthesia and curarisation are generally recommended to reduce O2 requirement and improve arterial oxygen saturation.
Preservation of normothermia is an essential part of the anaesthetic procedure: warmed room, insulating and warming cover, etc. This is particularly important for reducing the risk of pulmonary hypertensive crises and blood clotting disorders [8].

Regional versus general anaesthesia (non-cardiac surgery)
In a haemodynamically complex situation, there is often a tendency to consider regional anaesthesia as lower risk than general anaesthesia. While this is true of distal limb blocks, it is certainly not the case for neuraxial blockade, and particularly not for spinal anaesthesia. This entails three major drawbacks: it causes systemic arterial vasodilation, reduces preload and prevents the patient from being hyperventilated.
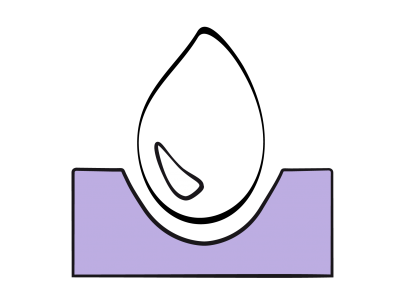
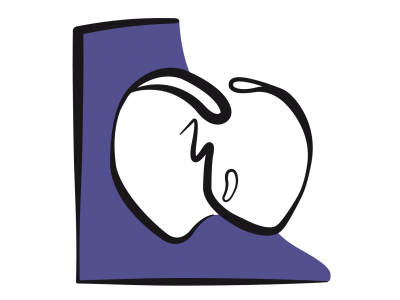
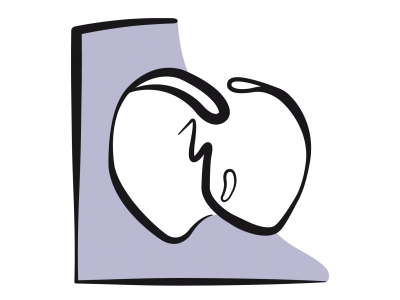
The fall in systemic vascular resistance exacerbates R-to-L shunts (Figure 15.61), bidirectional shunts with PAH or pulmonary stenosis, and L-to-R corrective shunts for low pulmonary blood flow (Figure 15.63). In contrast, it reduces L-to-R shunts as in ASDs or VSDs (Figure 15.62). Neuraxial blockade is therefore recommended for L-to-R shunting but not for R-to-L shunts, unless an epidural is performed with very gradual installation of sympathetic blockade. Spinal anaesthesia is strongly advised against for R-to-L shunts and severe obstructions of the left ejection pathway [2]. A high thoracic epidural (C7-T5) does not reduce PVR as there are few α1 receptors in the pulmonary vessels. Since sympathetic stimulation causes pulmonary vasodilation, PAP tends to rise if it is inhibited. Moreover, epidural causes cardiac sympatholysis, which reduces cardiac output and heart rate. Methaemoglobin formation due to prilocaine can be extremely dangerous for cyanotic patients [2].

Figure 15.61: Right → left shunt. This is increased when SVR falls, which lowers pulmonary arterial blood flow (Qp). The shunt is reduced if SVR increases and PVR falls. As a result, pulmonary flow increases and SaO2 improves.

Figure 15.62: Left → right shunt. This is increased if SVR rises and PVR falls, in which case pulmonary blood flow (Qp) is excessive. The shunt is reduced if SVR falls and PVR rises. This is the goal pursued by anaesthesia. Since LV compliance decreases with age, LV end-diastolic pressure tends to increase, raising LA pressure and reinforcing L-to-R shunting.

Figure 15.63: Left → right corrective shunt to increase pulmonary blood flow (Qp) in the event of pulmonary stenosis. Its blood flow decreases as SVR falls and also rises as SVR increases.
In the event of pulmonary hypertension or situations in which it is advisable to reduce PVR, general anaesthesia is preferable as it enables hyperventilation. This largely compensates for the risk of increasing RV afterload, since PVR is already so high (mean PAP 30-50 mmHg) that IPPV proportionally increases right ejection impedance very little (see Ventilation and PAH).
Target haemodynamic conditions and the authors’ recommendations for anaesthesia in grown-up congenital heart patients are summarised in Tables 15.9 and 15.10.


© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
Premedication
Hypoventilation and hypoxia are catastrophic in patients with low pulmonary blood flow or a cyanotic shunt. Premedication must therefore be moderate and not involve opiates. Preoperative fasting may cause dehydration in cyanotic patients whose Ht is high and whose blood hyperviscosity is an additional risk factor. These patients require a fluid infusion if they undergo surgery in the second part of the day or in very warm conditions. Grown-up congenital heart patients are highly dependent on their family and medical team. They are prone to panic in unknown environments or those perceived as hostile and stressful. This phenomenon affects Down syndrome patients in particular. It is therefore important to show them considerable empathy and ensure that they are unaware of the stress that their pathology causes the medical team.
Equipment
Vascular access points are often limited by previous operations and anatomical constraints. For example, arterial pressure is not measurable in the arm on the side of a Blalock-Taussig shunt, there is no venous access to the right heart after a Fontan procedure, and the IVC is often interrupted in heterotaxy syndrome. Compulsory precautions against the accidental injection of air into venous lines must be taken for any patients with shunts: aspiration of blood prior to injection, 3-way valves on all tubing, deairing of residual bubbles from syringes, injection with the syringe in a vertical position to ensure that bubbles remain attached to the plunger (Vidéo CD 3.0 Fig 15-58).
Monitoring
SpO2 is an extremely valuable indicator for congenital heart disease patients. A good signal must therefore be sought and the transducer must be positioned appropriately for the pathology. Variations in SVR and PVR, actual pulmonary blood flow and Qp/Qs ratio are clearly reflected in variations in arterial oxygen saturation (SaO2, SpO2) or tissue oxygen saturation (ScO2, cerebral oxygen saturation measured by near-infrared spectroscopy). However, these measurements provide no information on O2 delivery in the event of a large L-to-R shunt (Qp/Qs ≥ 3:1). In the event of R-to-L shunting, ETCO2 gives an underestimation of PaCO2 due to the dead-space effect of blood volume avoiding the lungs.
An invasive measurement of arterial pressure is the second most important monitoring procedure [5]. It provides instant data on SVR, blood volume and systolic function. Moreover, it enables frequent blood-gas analyses and rapid detection of metabolic acidosis. It is essential to set it up prior to induction.
Central venous access may be difficult in the event of a left-sided superior vena cava or atrial switch after TGA. Following a Glenn procedure, the catheter placed in the jugular or subclavian vein measures pressure in the right pulmonary artery. CVP is measured by femoral line. If the venous return is diverted to the PA as in the Fontan procedure, a central venous catheter actually measures the upstream pressure of the pulmonary circulation, which must be at least 15 mmHg. In these highly thrombogenic situations, the catheter must be left in place for as short a period as possible. Moreover, it is impossible to interpret filling pressures in terms of blood volume due to congenital heart disease patients’ highly unusual haemodynamic conditions. CVP or PAWP are not useful for determining whether patients are hypo- or hypervolaemic.
The Swan-Ganz pulmonary catheter is not the most important tool and its contribution is somewhat disappointing for congenital heart disease patients, as it only analyses the pulmonary circulation. However, pulmonary blood flow may be different to systemic blood flow (Qp/Qs ≠ 1). Although PAP measurement provides reassurance, it is rarely indicated except in cardiac surgery cases with pulmonary hypertension. Filling pressures are even less effective blood volume end points in congenital heart disease patients than in normal individuals, since they are primarily dependent on anatomopathological conditions. It may be impossible or dangerous to position the catheter in many cases: atrial switch (Mustard or Senning procedure), tight pulmonary stenosis (tetralogy of Fallot), Fontan circulation (no RV). If an ASD or R-to-L shunt is present, the catheter may switch to the left. Moreover, shunting or atrioventricular valve insufficiency skews calculations of cardiac output and of resistance. The situation is unclear in the event of significant tricuspid insufficiency (TI). It is generally accepted that transpulmonary thermodilution gives an underestimation of actual cardiac output in the event of TI. However, this only appears to hold true in situations with high blood flow, since the TI causes overestimation of CO in the event of low blood flow [9]. CO is also overestimated by thermodilution in the event of L-to-R shunting. Since pulmonary hypertension is generally combined with TI, Swan-Ganz gives an underestimation of CO if PAP is high.
The PiCCO system avoids pitfalls associated with a pulmonary catheter by assessing systemic blood flow. However, transpulmonary thermodilution is required in order to calibrate it and its calculation of cardiac output is also skewed by any shunting present. It is particularly useful postoperatively following cardiac surgery, i.e. after shunts have been corrected. If shunting is present, the Qp/Qs ratio, which is assessed by SaO2 and acid-base balance, is more significant than the absolute blood flow value.
Perioperative transesophageal echocardiography (TEE) is without doubt a less invasive and much more effective monitoring technique. It provides abundant data on patients’ anatomy, remodelling, pathophysiology, ventricular function and blood volume, which is particularly useful since filling pressure measurements are unreliable for assessing blood volume in congenital heart disease patients (see below Impact of TEE).
Cerebral monitoring techniques such as EEG, BIS, transcranial Doppler and cerebral O2 saturation (ScO2, NIRS Near-infrared spectroscopy) are important if deep hypothermia or total circulatory arrest are required for surgery. Detailed information regarding these procedures can be found in Chapter 18 (see Anaesthesia for aortic arch surgery, Monitoring) and is not provided here.
Induction
Ideally, a Valsalva manoeuvre should be performed prior to induction in order to test the impact of positive-pressure ventilation on arterial pressure (catheter in place before induction). Direct flow from the systemic venous return to the arterial line accelerates induction with intravenous agents if a R-to-L shunt is present. In contrast, induction and elimination of halogenated agents are slowed due to low pulmonary blood flow [11]. In the event of L-to-R shunting, induction with intravenous agents is slightly slowed. The effect on induction with halogenated agents is negligible [12]. In practice, such notions have little impact on the course of anaesthesia.
Induction agents are chosen based on the pathology's haemodynamic impact. However, many are associated with effects that render their use with congenital heart disease patients problematic: reduction of preload and SVR (propofol, midazolam), negative inotropic effect (thiopental, propofol), tachycardia (thiopental) [1]. Etomidate is without doubt the substance with the least harmful effects and is recommended for any complex cardiac anaesthesia. However, a fentanyl-midazolam combination is a good alternative. Nevertheless, midazolam considerably delays extubation unless it is used solely for sedation (1-3 mg). Fentanyl and sufentanil are the most stable and best controlled opiates. They dose-dependently block stress response and inhibit PAH crises. Although remifentanil is useful for brief procedures, the bradycardia it causes may be prohibitive. While ketamine is useful for children, it is inappropriate for adult haemodynamic conditions since it increases PVR and myocardial O2 requirement, and has a significant direct cardiodepressant effect, which is concealed by its sympathetic stimulation [13].
Anaesthetic procedure (Table 15.8)
It is simplest to maintain anaesthesia with a halogenated agent. Sevoflurane offers the greatest stability. Isoflurane is indicated for lowering SVR. Desflurane is contraindicated due to its sympathetic stimulation and its idiosyncrasy of causing an increase in PVR if the inspired concentration is changed [6,7]. Ketamine and N2O have a pulmonary hypertensive effect in adults. Deep anaesthesia and curarisation are generally recommended to reduce O2 requirement and improve arterial oxygen saturation.
Preservation of normothermia is an essential part of the anaesthetic procedure: warmed room, insulating and warming cover, etc. This is particularly important for reducing the risk of pulmonary hypertensive crises and blood clotting disorders [8].
Regional versus general anaesthesia (non-cardiac surgery)
In a haemodynamically complex situation, there is often a tendency to consider regional anaesthesia as lower risk than general anaesthesia. While this is true of distal limb blocks, it is certainly not the case for neuraxial blockade, and particularly not for spinal anaesthesia. This entails three major drawbacks: it causes systemic arterial vasodilation, reduces preload and prevents the patient from being hyperventilated.
The fall in systemic vascular resistance exacerbates R-to-L shunts (Figure 15.61), bidirectional shunts with PAH or pulmonary stenosis, and L-to-R corrective shunts for low pulmonary blood flow (Figure 15.63). In contrast, it reduces L-to-R shunts as in ASDs or VSDs (Figure 15.62). Neuraxial blockade is therefore recommended for L-to-R shunting but not for R-to-L shunts, unless an epidural is performed with very gradual installation of sympathetic blockade. Spinal anaesthesia is strongly advised against for R-to-L shunts and severe obstructions of the left ejection pathway [2]. A high thoracic epidural (C7-T5) does not reduce PVR as there are few α1 receptors in the pulmonary vessels. Since sympathetic stimulation causes pulmonary vasodilation, PAP tends to rise if it is inhibited. Moreover, epidural causes cardiac sympatholysis, which reduces cardiac output and heart rate. Methaemoglobin formation due to prilocaine can be extremely dangerous for cyanotic patients [2].
Figure 15.61: Right → left shunt. This is increased when SVR falls, which lowers pulmonary arterial blood flow (Qp). The shunt is reduced if SVR increases and PVR falls. As a result, pulmonary flow increases and SaO2 improves.
Figure 15.62: Left → right shunt. This is increased if SVR rises and PVR falls, in which case pulmonary blood flow (Qp) is excessive. The shunt is reduced if SVR falls and PVR rises. This is the goal pursued by anaesthesia. Since LV compliance decreases with age, LV end-diastolic pressure tends to increase, raising LA pressure and reinforcing L-to-R shunting.
Figure 15.63: Left → right corrective shunt to increase pulmonary blood flow (Qp) in the event of pulmonary stenosis. Its blood flow decreases as SVR falls and also rises as SVR increases.
In the event of pulmonary hypertension or situations in which it is advisable to reduce PVR, general anaesthesia is preferable as it enables hyperventilation. This largely compensates for the risk of increasing RV afterload, since PVR is already so high (mean PAP 30-50 mmHg) that IPPV proportionally increases right ejection impedance very little (see Ventilation and PAH).
Target haemodynamic conditions and the authors’ recommendations for anaesthesia in grown-up congenital heart patients are summarised in Tables 15.9 and 15.10.
| Anaesthesia for GUCH patients |
|
Perioperative risk factors (by order of seriousness)
- PAH (mean PAP > 25 mmHg) - Cyanosis (SaO2 < 85%) - Reoperation, sequelae of previous surgery - Arrhythmias - Ventricular dysfunction - Severe obstruction of the ejection pathway Essential monitoring - ECG, SpO2, if possible ScO2 - Arterial catheter (position dependent on the pathology) in place prior to induction - Transesophageal echocardiography General anaesthesia - Preferred technique for non-cardiac surgery - Specific indications: R-to-L shunt, palliative shunt, PAH, obstruction - Hyperventilation, alkalosis, low ventilation P - Induction: etomidate, midazolam, slow propofol if stable heart disease - Maintenance: sevoflurane (isoflurane to lower SVR), propofol - Fentanyl or sufentanil, remifentanil if bradycardia tolerated - Contraindicated if PAH: ketamine, N2O, desflurane - Maintain preload and normothermia rigorously Neuraxial blockade - Maintain preload - Specific indication: L-to-R shunt - R-to-L shunt: spinal anaesthesia not advised, epidural possible with very gradual installation of sympathetic blockade |
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
- ANDROPOULOS DB. Anesthetic agents and their cardiovascular effects, In: ANDROPOULOS DB, STAYER SA, RUSSEL IA. Anesthesia for congenital heart disease. Oxford: Blackwell Publishing, 2005, 49-63
- BAEHNER T, ELLERKMANN RK. Anesthesia in adults with congenital heart disease. Curr Opin Anaesthesiol 2017; 30:418-25
- BAUMGARTNER H, BONHOEFFER P, DE GROOT NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915-57
- BHATT AB, FOSTER E, KUEHL K, et al. Congenital hesart disease in older adult. A Scientific Statement from the American Heart Association. Circulation 2015; 131:1884-931
- CHASSOT PG, BETTEX DA. Anesthesia and adult congenital heart disease. J Cardiothorac Vasc Anesth 2006; 20:414-37
- CIOFOLO M, REIZ S. Circulatory effects of volatile anesthetic agents. Minerva Anaesthesiol 1999; 65:232-8
- EBERT TJ, MUZI M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers: a comparison with isoflurane. Anesthesiology 1993; 79:444-53
- FRANK SM, FLEISHER LA, BRESLOW MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277: 1127-34
- HEERDT PM, BLESSIOS GA, BEACH ML, et al. Flow dependency of error in thermodilution measurement of cardiac output during acute tricuspid regurgitation. J Cardiothorac Vasc Anesth 2001; 15:183-7
- HO VB. Radiologic evaluation of suspected congenital heart disease in adults. Am Fam Physician 2009; 80:597-602
- HUNTINGTON JH, MALVIYA S, VOEPEL-LEWIS T, et al. The effect of a right-to-left intracardiac shunt on the rate of rise of arterial and end-tidal halothane in children. Anesth Analg 1999; 88:759-62
- LAIRD TH, STAYER SA, RIVENES SM, et al. Pulmonary-to-systemic blood flow ratio effects of sevoflurane, isoflurane, halothane and fentanyl/midazolam with 100% oxygen in children with congenital heart disease. Anesth Analg 2002; 95:1200-6
- LOVELL AT. Anaesthetic implications of grown-up congenital heart disease. Br J Anaesth 2004; 93:129-39
15. Anesthesia for adult congenital heart disease patients
- 15.1 Introduction
- 15.2 Nomenclature and pathophysiology
- 15.3 Approach by pathology
- 15.3.1 Classification
- 15.3.2 Diagnostic methods
- 15.3.3 Anomalous venous returns
- 15.3.4 Atrial septal defects (ASDs)
- 15.3.5 Atrioventricular canal (AVC) defects
- 15.3.6 Ebstein anomaly
- 15.3.7 Ventricular septal defects (VSDs)
- 15.3.8 Ventricular hypoplasia
- 15.3.9 Tetralogy of Fallot
- 15.3.10 Mixed shunt
- 15.3.11 Pulmonary stenosis
- 15.3.12 Anomalies of the LV ejection pathway
- 15.3.13 Transposition of the great arteries (TGA
- 15.3.15 Coarctation of the aorta
- 15.3.14 Congenitally corrected TGA
- 15.3.16 Arterial abnormalities
- 15.4 General considerations for anesthesia
- 15.5 Conclusions